Unwanted immunogenicity is a significant issue affecting most biotherapeutic products, including biosimilars. Anti-Drug Antibodies (ADA) can be associated with adverse reactions and can impact clinical efficacy and PK/PD profile. Neutralizing antibodies can bind to the active part of the molecule thereby blocking the therapeutic effect of the drug, and may inhibit the activity of corresponding endogenous factor. There is a clear need to be able to detect those potential culprits. Prediction, minimizing and assessment of immunogenicity are challenges facing the biotechnology industry.
The industry standard, regulatory acceptable testing strategy for ADA is step-wise: screening for total ADA, confirmation, and neutralizing activity testing. The screening step is a cut point assay set up to allow 5% false positives. The confirmation step, in which the binding of ADA is competed out by addition of excess of free drug, confirms the presence of specific antibodies and allows to focus on true positives. These are usually tittered and evaluated for potential neutralizing activity using biological activity assays, which provide a functional biological system to assess if the antibodies detected by the immunoassay have neutralizing capability. The common practice is to adapt the drug's release potency assay for this purpose, but binding and even cell free assays can be used when cell based potency assays cannot be adapted for this purpose.
There is a critical need to understand sensitivity and specificity of ADA assays. Sensitivity refers to the ability of an assay to detect very small concentrations of abs in a test sample. A highly sensitive assay is more likely to detect low levels of specific abs and therefore has a low probability of producing false negative results. Sensitivity is affected by many factors (type of drug, affinity of antibody, serum, methodology, technology, drug interference). Specificity refers to the ability of an assay to detect only those antibodies directed against the specific target protein. The specificity of each assay is a product of the biochemical mechanics of antibody binding to its ligand and the subsequent detection methodology used. A highly specific assay has a very low probability of measuring false positives resulting from non-specific binding.
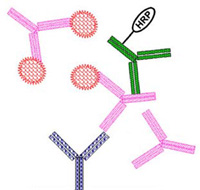 One of the major problems to address when designing an ADA assay for a biologic is free drug interference. Biologics have long half-lives, resulting in high concentrations in circulation for a relatively long time. The ADAs form complexes with the free drug, thereby hindering detection.
One of the major problems to address when designing an ADA assay for a biologic is free drug interference. Biologics have long half-lives, resulting in high concentrations in circulation for a relatively long time. The ADAs form complexes with the free drug, thereby hindering detection.
To overcome this problem an appropriate study design is of paramount importance. Not less cardinal is the use of the best fitted methodologies and technological platforms in assay design.