One of the most critical factors in developing biopharmaceuticals today is ensuring that the bioassays and the bioanalytical test methods generate meaningful data. The US Food and Drug Administration (FDA), United States Pharmacopeia (USP) and the International Conference on Harmonization (ICH), have each recognized the importance of this to the drug development process and have separately increased validation requirements in recent years.
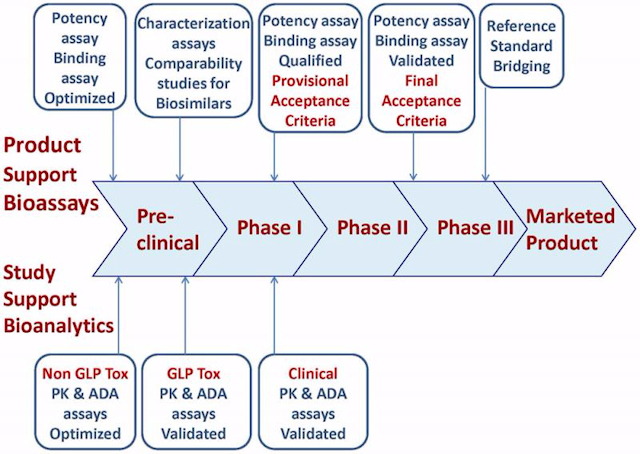
Bioassays are used for lot release, stability, and/or characterization. The development of both new biologics and biosimilars mandates potency assay, as well as additional bioassays development for assessment of biological activity. Comparison of biotherapeutic products before and after manufacturing changes, as well as comparability of biosimilars is required to show that the products are highly similar. Intricate bioanalytical methodology needs to be in place for pharmacokinetics (PK) and anti-Drug antibodies (ADA) determination in pre-clinical and clinical studies.
 Bioassays are the foundation by which analytical control strategies for biologics are built. Bioassays are an operationally challenging class of test methods due to their reliance on a biological substrate (e.g. animals, living cells, or functional complexes of target receptors). Because of multiple factors arising from this reliance on biology, they typically exhibit a greater variability than do chemically-based tests. These are often cell based assays, requiring special attention to cell banking and maintenance.
Bioassays are the foundation by which analytical control strategies for biologics are built. Bioassays are an operationally challenging class of test methods due to their reliance on a biological substrate (e.g. animals, living cells, or functional complexes of target receptors). Because of multiple factors arising from this reliance on biology, they typically exhibit a greater variability than do chemically-based tests. These are often cell based assays, requiring special attention to cell banking and maintenance.
The ability of the assay to characterize and demonstrate biological activity is essential. Because molecules can be very complex or have multiple modes of action, companies are challenged with developing assays that are biologically relevant for the analysis of different mechanisms. Bioassays are also used to determine the ability of anti-drug antibodies to neutralize the biological effect of the drug in a cell based system. Immunoassays, also referred to as ligand-binding or activity-binding assays, are applied throughout the bioprocess development and production. Bioanalytical tools and techniques continue to evolve and significant scientific and regulatory experience in their application is being gained. Bioassay validation requires meticulous assessment of accuracy, precision, range of quantification, dilutional linearity, parallelism, specificity and selectivity, ruggedness and robustness of the method, as well as potential matrix effect, sample and solution stability, and more.
NCBiologics Consulting provides: scientific input, oversight, and study execution regarding design, conduct and interpretation of lab experiments to evaluate biotherapeutics biological activity, pharmacokinetics, and immunogenicity. Natalie can assist your company in the preparation of appropriate development plans in the Chemistry, Manufacturing and Controls (CMC), preclinical and clinical aspects, and international registration strategy, in bioassay development and validation done in house, or by identifying and managing the right CRO.